Exploring the realm of Pfizer Side Effects Monitoring and Safety Reporting opens up a world of crucial information. Delve into this guide that promises an enriching journey through the intricacies of monitoring and reporting on Pfizer medications.
Uncover the nuances of safety reporting processes, stakeholder involvement, and the meticulous strategies employed by Pfizer to ensure the safety and efficacy of its products.
Pfizer Side Effects Monitoring and Safety Reporting
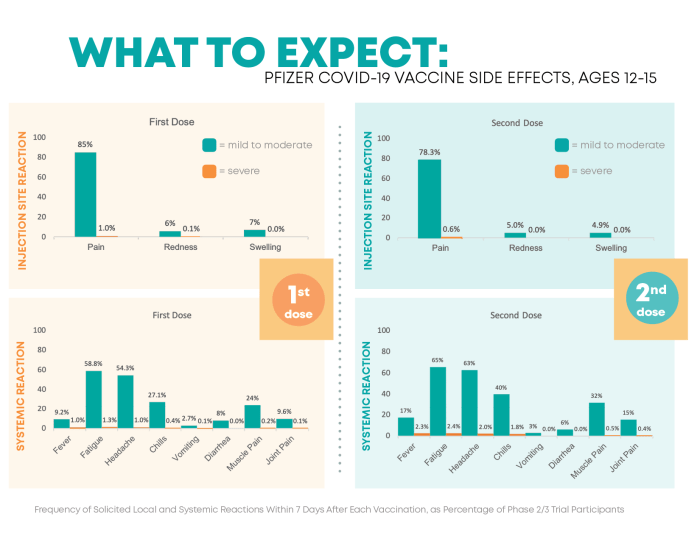
Monitoring side effects in Pfizer medications is crucial for ensuring the safety and efficacy of the products. By actively tracking and analyzing potential side effects, Pfizer can take necessary actions to protect consumer health and well-being.Overview of Safety Reporting Process
- Pfizer's safety reporting process involves collecting data on adverse events associated with their medications.
- Healthcare professionals and consumers can report any observed side effects to Pfizer through designated channels.
- Pfizer evaluates these reports to assess the potential risks and benefits of their products.
Key Stakeholders in Monitoring Pfizer Side Effects
- Healthcare providers play a vital role in monitoring and reporting side effects to Pfizer.
- Regulatory agencies such as the FDA and EMA collaborate with Pfizer to oversee drug safety.
- Pfizer's internal pharmacovigilance team actively monitors and analyzes safety data to identify potential risks.
Safety and Efficacy Assurance by Pfizer
- Pfizer conducts clinical trials to assess the safety and efficacy of their products before market approval.
- Post-market surveillance helps Pfizer to continuously monitor and evaluate the real-world safety profile of their medications.
- Regular safety updates and risk management plans are implemented by Pfizer to address any emerging safety concerns.
Pharmacovigilance Systems
Pharmacovigilance is the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. In the context of Pfizer, pharmacovigilance plays a crucial role in monitoring the side effects of Pfizer medications, ensuring patient safety, and complying with regulatory requirements.Pfizer's Pharmacovigilance Systems vs Industry Standards
Pfizer's pharmacovigilance systems are known for their robustness and adherence to industry standards. They have well-established processes for collecting, analyzing, and reporting safety data. Pfizer goes beyond the minimum regulatory requirements to ensure comprehensive monitoring of their medications, setting a high bar for safety reporting in the pharmaceutical industry.Methodologies Used in Pharmacovigilance for Pfizer Medications
- Pfizer employs a multi-tiered approach to pharmacovigilance, including proactive monitoring of adverse events through clinical trials, post-marketing surveillance, and real-world data analysis.
- The company utilizes cutting-edge technology and data analytics to identify potential safety signals early, allowing for timely intervention and risk mitigation.
- Pfizer also fosters a culture of continuous learning and improvement, regularly updating their pharmacovigilance processes based on emerging trends and best practices.
Successful Safety Reporting Outcomes in Pfizer's Pharmacovigilance Practices
- One notable success story in Pfizer's pharmacovigilance practices is the rapid identification and reporting of a rare but serious side effect associated with one of their medications. Thanks to their vigilant monitoring systems, appropriate risk communication measures were promptly implemented, minimizing harm to patients.
- Another example is Pfizer's proactive approach to safety reporting, where they actively engage with healthcare providers and patients to encourage reporting of any potential adverse events. This collaborative effort has led to enhanced pharmacovigilance outcomes and improved patient safety.
Adverse Event Reporting
 Adverse event reporting is a critical component of monitoring the safety and efficacy of Pfizer drugs. When patients, healthcare professionals, or any other individual experiences an adverse event or side effect while using a Pfizer product, it is essential to report this information to Pfizer's pharmacovigilance system.
Adverse event reporting is a critical component of monitoring the safety and efficacy of Pfizer drugs. When patients, healthcare professionals, or any other individual experiences an adverse event or side effect while using a Pfizer product, it is essential to report this information to Pfizer's pharmacovigilance system.Process of Adverse Event Reporting for Pfizer Drugs
- Healthcare professionals or patients report adverse events to Pfizer through various channels, such as online portals, phone hotlines, or direct communication with Pfizer representatives.
- Pfizer collects and evaluates the reported adverse events to assess the potential risks associated with their products.
- Once the adverse event is confirmed, Pfizer takes appropriate actions, which may include updating product labels, issuing safety alerts, or conducting further investigations.
Regulatory Requirements for Reporting Adverse Events
- Regulatory authorities, such as the FDA and EMA, mandate that pharmaceutical companies like Pfizer report all adverse events associated with their products within specific timelines.
- Pfizer must adhere to strict guidelines and regulations to ensure timely and accurate reporting of adverse events to regulatory authorities.
Challenges and Limitations of Adverse Event Reporting
- Underreporting of adverse events by healthcare professionals and patients can lead to incomplete safety data and hinder the detection of potential risks.
- Difficulty in determining causality between the adverse event and Pfizer products, especially in cases of multiple medications or underlying health conditions, can pose challenges in reporting.
Strategies for Improving Adverse Event Reporting Accuracy and Efficiency
- Enhancing healthcare professionals' awareness and education about the importance of adverse event reporting can encourage more comprehensive reporting.
- Implementing user-friendly reporting systems and tools can streamline the reporting process and increase efficiency.
- Continuous monitoring and evaluation of reported adverse events can help Pfizer identify trends and take proactive measures to ensure patient safety.
Risk Management Strategies
When it comes to monitoring and mitigating side effects, Pfizer employs a comprehensive set of risk management strategies to ensure the safety of its medications. These strategies involve assessing and prioritizing risks associated with each medication, as well as implementing risk minimization action plans as part of its safety reporting processes. Pfizer has a strong track record of successfully managing risks and enhancing the safety profile of its products.Assessment and Prioritization of Risks
- Pfizer conducts thorough assessments of potential risks associated with its medications throughout the drug development process.
- The company utilizes data from clinical trials, post-marketing surveillance, and real-world evidence to evaluate the safety profile of its products.
- Risks are prioritized based on the severity of potential side effects, the likelihood of occurrence, and the impact on patient outcomes.
Role of Risk Minimization Action Plans
- Pfizer develops risk minimization action plans to proactively address identified risks and enhance the safe use of its medications.
- These plans may include additional monitoring, educational initiatives for healthcare professionals and patients, label updates, or restricted distribution programs.
- By implementing these action plans, Pfizer aims to minimize the potential harm associated with its products and improve patient safety.
Examples of Successful Risk Management Strategies
- Pfizer's implementation of REMS (Risk Evaluation and Mitigation Strategies) for certain medications has been effective in managing risks and ensuring safe use.
- The company's collaboration with regulatory agencies and healthcare providers to address emerging safety concerns has led to timely risk mitigation measures.
- Pfizer's commitment to transparent communication about potential risks and safety updates has fostered trust among patients and healthcare professionals.
Closing Notes
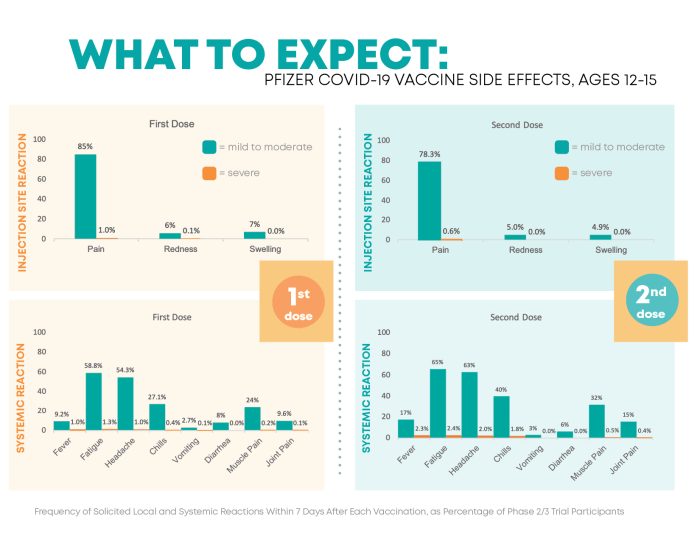
In conclusion, this guide has shed light on the vital aspects of Pfizer Side Effects Monitoring and Safety Reporting. From understanding pharmacovigilance systems to exploring risk management strategies, the significance of monitoring side effects in Pfizer medications is unmistakable. Stay informed and empowered with this essential knowledge.
Expert Answers
What is the importance of monitoring side effects in Pfizer medications?
Monitoring side effects helps in assessing the safety and efficacy of Pfizer drugs, ensuring patient well-being.
How does Pfizer ensure the safety and efficacy of its products through monitoring and reporting?
Pfizer employs rigorous monitoring processes and collaborates with key stakeholders to maintain high safety standards.
What are the regulatory requirements for reporting adverse events associated with Pfizer products?
Regulatory requirements mandate timely and accurate reporting of adverse events linked to Pfizer medications.
How does Pfizer assess and prioritize risks associated with its medications?
Pfizer utilizes risk management strategies to evaluate and address potential risks, prioritizing patient safety.
What are some successful risk management strategies implemented by Pfizer in the past?
Pfizer has implemented risk minimization action plans and proactive monitoring to ensure effective risk management.